 Q 1.
Q 1.
What are the similarities and differences between a neutron and a proton?
|
In what ways do electrons differ from protons? |
|
What is a "nucleon"? |
|
What determines the chemical behavior of an atom? |
|
What is an ion? |
|
My book says that oxygen (symbol O) has an atomic mass of 15.9994. What does that mean? How can you get "non-whole numbers" of atomic mass when all that mass is made of nucleons which are whole numbers? |
|
What are the number of neutrons and protons in oxygen-15 (15O)? |
|
What is an alpha particle and what is its charge? |
|
What is a beta particle and what is its charge? |
|
What is a gamma ray and what is its charge? |
|
Which radiation are involved in transmutation? |
|
Is atomic mass effected by transmutation? |
|
What's a "Dalton" and how is it used in atomic mass? |
|
Describe the isotopes of hydrogen. |
|
Define atomic number, atomic mass and relative atomic mass. |
|
In the 20th century man learned how to slam tritium atoms together, causing nuclear fusion, the same process that occurs in the sun. The energy is released so quickly that this nuclear reaction is referred to as a bomb - a hydrogen bomb! Each H-bomb is designed to carry a specific amount of tritium in order to produce a specific amount of fusion (when detonated). However, because of tritium's beta decay, all H-bombs must be dismantled at regular intervals and fresh tritium added. Recall NOTHING, not even the military, can stop the constant decay of radioisotopes! Consider an H-bomb which requires a minimum of 1 kilogram of tritium for a "proper" detonation. What is the minimum amount of tritium which must be packed into this bomb for it to work (able to be detonated) for 36 years? (Recall that tritium has a half-life of 12 years.) |
|
Radioisotope "X" has an atomic mass of 224 and decays into another element with an atomic mass of 220. What particle is emitted in the decay of radioisotope X, how would you protect yourself from it, and how does it become another element? |
|
A nickel (5 cent piece from the USA) weighs about 5 grams. (Isn't that convenient?) Assume a nickel was made radioactive (in a nuclear explosion or placed inside a nuclear reactor) and was found to give off 74 billion disintegrations each second. (You would not want this coin in your pocket!). |
|
Where did nitrogen (atomic number 7) come from? |
|
Where did gold (atomic number 79) come from? |
|
What is the difference between the carbon in your body and the carbon in a distant star? |
| What are the first three electron shells and their maximum electron occupancy? In what way does this effect how they are "filled"? What do shells tell us about the atom? |
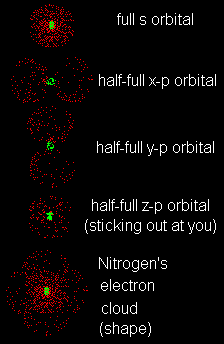
| Nitrogen (N) has an atomic number of 7. Describe the orbitals of a nitrogen atom. Take your time. Work carefully and slowly. |
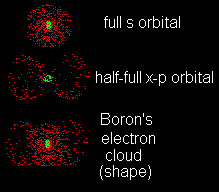
| Boron (B) has an atomic number of 5. Do with boron the same things you did for nitrogen (above). |
| You'll recall that M-shells can hold up to 18 electrons. |

Arthur's (and Merlin's) Answers
|
Neutrons and protons have almost the same mass. (But neutrons are slightly heavier.) |
|
Electrons have a negative charge (-1) but protons have a positive charge (+1). |
|
The term "nucleon" refers to protons and neutrons. NOTE: Don't get neutrons and nucleons mixed up. Neutrons are nucleons, but so are protons! |
|
The number of protons determines the chemical behavior of an atom. |
|
An ion is an atom with a (net) charge. This is caused by it having more or fewer protons than electrons. If you add up all the protons and subtract all the electrons (because protons are +1 and electrons -1) you find the atom's (net) charge. If it is anything but zero, it's an ion! |
|
Oxygen is listed as having an atomic mass of 15.994 because it represents a MIX of oxygen isotopes. Most oxygen atoms in the universe have 8 protons and 8 neutrons, so an atomic mass of 16. But there must be some oxygen atoms in the universe with an atomic mass of less than 16 Daltons (probably 15 Daltons). |
|
Oxygen-15 (15O) must have 8 protons, in order for it to be oxygen. (I reminded you that oxygen has 8 protons, in Answer 6.) If it had 7 protons it would not be oxygen, but something else (it would be nitrogen, but you may not have remembered that). |
|
An alpha particle is a helium nucleus. |
|
A beta particle is an electron, so it has a charge of -1. |
|
A gamma ray is a powerful beam of light (too powerful to be seen but very capable of causing damage). |
|
Transmutation is the transformation of one atom or element into another. This requires a change in the number of protons. When an atom spits out an alpha particle, it loses two protons (and one or two neutrons) so it must become another element. When an atom spits out an electron it does so because it has created a new proton from a neutron. (It's not magic but it sure is close!). It spits out the electron (beta particle) to conserve charge. Oh, by the way, atoms often loose their electrons without involving beta decay. They may pick up a lot of energy (heat) from their environment and simply toss away some of their electrons. This is ionization, not beta decay. Beta decay is a "nuclear transformation event". It starts in the nucleus. Ionization is an "orbiting electron event" and it has to do with the electrons already around the atom (and has nothing to do with transmutation or the nucleus). Gamma rays are sometimes produced during transmutations of elements we haven't yet discussed, but they are not (directly) involved in transmutation. |
|
Yes and no. |
|
A Dalton is a unit of atomic mass (not weight). Hydrogen is the fundamental unit and has a mass of one Dalton. A better way to look at it is to say that each nucleon has a mass of one Dalton. We calculate the atomic mass by adding up the number of nucleons and express that number as Daltons. |
|
Hydrogen has three "common" isotopes. |
|
Atomic number is the number of PROTONS in the atom. It defines the element and determines its chemical properties and behavior. Each element has one (and only one) atomic number. Atomic mass is the number of NUCLEONS in the atom. Each nucleon has a mass of one Dalton. Each element can have a variety of isotopes, each differing by the numbers of neutrons and causing it to have a different atomic mass. Relative atomic mass is the AVERAGED mass of all of an element's isotopes. The relative proportions of an element's isotopes are calculated along with the atomic mass of each isotope. The relative atomic mass is a convenient way to represent the mass and abundance of all that element's isotopes, all in a single number. |
|
This question asks you to calculate the amount of tritium needed to leave 1 kilogram of tritium available (for the bomb) in 36 years. Tritium has a half-life of 12 years, so in 36 years it will have gone through 3 half-lives. Therefore, 8 kilograms of tritium must be packed into the bomb so there will be 1 kilogram undecayed in 36 years. NOTE: The answer is not 3 kilos. Some folks get confused about the half-life math. They think you can multiply the number of half-lives by the amount needed at that time, in order to figure out what you need to start with. This is wrong because the math is not used correctly. (Students with advanced math education will recognize that half-lives are an EXPONENTIAL function, not a LINEAR one. And they will use exponetials to arrive at the same answer more quickly than the method described here.) Working backward through time: Just to be sure, check this answer by working the problem out in the other direction: |
|
For an atom to change from an atomic mass of 224 to 220, it must lose 4 nucleons. |
|
There are lots of ways to do the math. Arthur's way goes like this: I agree with Arthur, but like to write it all out in a long equation (because it helps me keep track of the units).
|
|
Nitrogen was created inside a star billions of years ago. After the star had used up all its hydrogen (creating helium), it underwent a contraction, which started a new type of nuclear fusion. There may have been other combinations of atoms fused together that can make nitrogen, but the end result is a nucleus with 7 protons. That's nitrogen. |
|
Gold, with an atomic number of 79, has 79 protons. This is far too many for mere nuclear fusion inside a star. This requires a huge explosion of the star, a nova, to smash heavy iron nuclei together. There are lots of different combinations of fusion and decay which could make gold. Perhaps you've thought of others. It doesn't matter exactly which combination you used. Just so long as the math worked out. |
|
NOTHING! |
| The inner most shell is the K-shell and it holds only 2 electrons. The next shell is the L-shell which can hold no more than 8 electrons. This is followed by the M-shell which can hold up to 18 electrons. Electrons are "assigned" to the inner shells first and one works outward, successively filling shells, until all the electrons are used up. Shells define the size of an atom by showing us where the outermost shell lays. That's the largest cloud and therefore represents the outer boundary of an atom. |
| The nitrogen atom has 7 electrons (because it isn't an ion). You must distribute them. First among the (principle) shells and then among the orbitals (subshells). First the shells. Now do the orbitals (subshells). All shells, including the K-shell, have an s orbital. In fact, the K-shell ONLY has an s orbital. So the two electrons in the K-shell are in an s orbital. This is a sphere. An inner sphere. The next shell, the L-shell, has an s orbital (obviously) and p orbitals. The orbitals of this outer shell will give the atom its shape. The remaining 5 electrons must be assigned to them. This makes things more complex. The first 2 electrons go into the L-shell's s orbital because s orbitals are of lower energy than p orbitals so electrons fill them first. The remaining 3 electrons go into the (higher energy) p orbitals. But which ones? There are three p orbitals to choose from; x, y and z. If you put one electron into each orbital you did it right! You remembered Hund's rule and used it correctly. What about spins? There are two spin rules to recall. Hund's rule tells you that when orbitals are "half full" (have only one electron in each) they should all be parallel. That means they should have the same spins. Therefore, all three electrons in nitrogen's p orbitals are either all "up" or all "down". The other spin rule comes from Pauli. He said that when two electrons share an orbital, they must have opposite spins, one "up" and the other "down". In nitrogen, the K-shell s orbital and L-shell s orbital are full, so both will have pairs of electrons with opposite spins.
|
| Boron has 5 protons so it must have 5 electrons. The two electrons in boron's K-shell are in an s orbital (the only kind of orbital the K-shell can have) and they have opposite spins. They have nothing to do with the atom's size or shape. It is the outer shell that tells us that.
If this atom had one more electron (like carbon), it would have placed it in another orbital to give two pairs of half-full lobes at right angles to each other. (And those two electrons would have had parallel spins.) |
 A 25.
A 25.
If you guessed that M-shells have d orbitals, you are right!
(Don't be disappointed if you missed that one. This is new stuff).
The M-shell has an s orbital (because all shells have an s orbital) which hold two electrons.
It also has the normal types of p orbitals (x-p, y-p and z-p) which can hold another six electrons.
That leaves 10 electrons to go somewhere.
Perhaps you remembered that there are five different types of d orbitals. We didn't talk about them, but you may remember that ANY orbital can hold up to two electrons. That means an entire set of d orbitals can hold ten electrons.
Therefore a full M-shell would have
2 electrons in the single s orbital,
6 electrons in three p orbitals, and
10 electrons in the five d orbitals.
That gives you a total of 18 electrons that can find a home in the M-shell.
Don't feel bad if you missed that. But do try to understand my explanation.
A funny thing about these larger shells is that they can hold more electrons, but they don't like to! They don't like to use their d and f orbitals!
For example, calcium (Ca) has 20 electrons (because it has an atomic number of 20). If you were assigning calcium's electrons to shells and orbitals you would expect the electrons to be placed like this...
K-shell gets 2 electrons in its s orbital (and it does),
L-shell gets 8 electrons in its s and three p orbitals (and it does).
M-shell gets the remaining 10 electrons in its orbitals, BUT IT DOESN'T!
There's plenty of room for those ten electrons in calcium's M-shell.
Calcium puts two electrons into its M-shell s orbital (like it should) and six electrons into its M-shell p orbitals (filling all three types). You would expect the remaining two electrons to go into d orbitals (whatever they are shaped like). Instead, calcium puts them into the NEXT shell, the N-shell!
Isn't that odd?
So the electronic configuration of calcium is ...
K-shell has 2 electrons in its s orbital.
L-shell has 2 electrons in its s orbital and 6 in its three p orbitals.
M-shell has 2 electrons in its s orbital and 6 in its three p orbitals.
N-shell has 2 electrons in its s orbital.
Weird!
I'm pointing this out because many students stumble across it.
In the large atoms, electrons are often assigned into the next shell's s orbital before the d orbital have been touched. And the even larger atoms which have f orbitals to work with don't use them until they have filled both the s AND p orbitals of the next larger shell.
If you think this has to do with some part of quantum mechanics we haven't discussed, you'd be right!
No comments:
Post a Comment